Aug 30, 18 · When the Gelucire % increased from 1 to 3% in the SLN formulation with the same levels of X 1 and X 3, Y 5 decreased from 1125 to 850 h in F6 and F1, respectively;May 10, 18 · The bioavailability of another HIV protease inhibitor, ritonavir, was also substantially enhanced, relative to a conventional formulation, by using a solid dispersion formulation prepared from a mixture of Gelucire 50/13, polysorbate 80 and polyoxyl 35 castor oil (32)Gelucire 50/13 based SD, prepared by SM remarkably increased the dissolution within 15 min (8727 ± 225%) and was supported by dissolution parameters (Q 15, IDR, RDR, % DE, f 1, f 2) These SDs showed pHdependent solubility

Improvement In Solubility Using Poloxamer 1 And Gelucire 50 13 A Case Study Patra Ch Niranjan Swain Suryakanta Panda Tapan Kumar Amazon Com Books
Gelucire 50/13 hlb
Gelucire 50/13 hlb-Gelucire 5013,CAS,,Gelucire 5013 suppliers Approved Bulk Manufacturers Want to be listed as an approved manufacturer (Free service but requires approvement)?Hydrophilicity and low density, Gelucire 50/13 may be considered an appropriate carrier for designing fast release floating drug delivery system11 Gelucire containing only glycerides or a mixture of glycerides and PEG esters (Gelucire 39/01, 43/01) are used in the preparation of sustained release formulation



Improvement In Solubility Using Poloxamer 1 And Gelucire 50 13 A Case Study Patra Ch Niranjan Swain Suryakanta Panda Tapan Kumar Amazon Com Books
Jul 08, 11 · The ability of Gelucire 50/13 to yield NLC was evaluated by using Precirol ATO 5 as a model solid lipid and various liquid lipids (oils) Gelucire 50/13 based NLC (GeluPearl) were evaluated for their ability to improve the efficacy of RPGVisit ChemicalBook To find more Gelucire 5013() information like chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight, physical properties,toxicity information,customs codes You can also browse global suppliers,vendor,prices,Price,manufacturers of Gelucire 5013() At last,GelucireGelucire 50/13 and poloxamer 1 based solid dispersions respectively The Gibbs free energy ΔG tr ° values were all negative for gelucire 50/13 (0, 01, 025, 05, 075, 1, 2, 4, 6, 8 and 10 % w/v) and poloxamer 1 (0, 01, 025, 05, 075 and 1 % w/v) indicating spontaneous nature of solubilisation FTIR and DSC spectra showed In vitro
Jul 23, · Gelucire 50/13 (Stearoyl polyoxyl32 glycerides) exhibited highest solubilization capability among the tested lipid excipients Therefore, Gelucire 50/13 was chosen for the nanoencapsulation ofGelucire 50/13 is an excipient composed of fatty acid (C16 and C18) esters of glycerol, PEG esters and free PEG It melts at approximately 50°C and hasJul , 14 · Solid dispersion (SD) system of everolimus (EVR) with Gelucire 50/13 (Stearoyl polyoxyl32 glycerides) was prepared using melt granulation technique with the aim of improving the physicochemical properties and dissolution rate
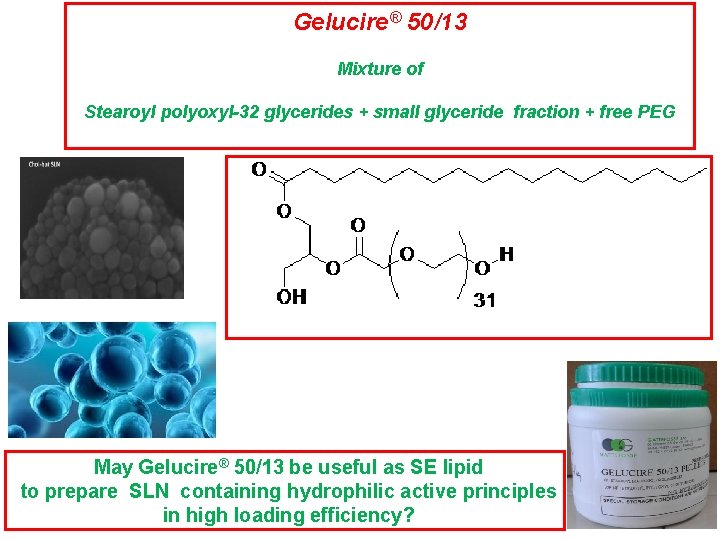
Jun 18, 18 · Gelucire® 50/13 is a stearoyl polyoxyl/macrogol 32 glycerides NF/EP and consists of mono, di and triglycerides and PEG32 (MW 1500) mono and diesters of palmitic (C16) and stearic (C18) acids It is available in pellet form HAVE A LOOK AT OUR NEWEST GELUCIRE ARTICLE HEREAnd from 1025 to 775 h in F9 and F8, respectively This result could be owing to the decrease in particle size as GelucirePreparation and in vitro evaluation of AllopurinolGelucire 50/13 solid dispersions



Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents



Spray Congealing An Emerging Technology To Prepare Solid Dispersions With Enhanced Oral Bioavailability Of Poorly Water Soluble Drugs Abstract Europe Pmc
Developed to melt within specific ranges Also Known As G 5013 Networked 0 relevant articles (0 outcomes, 0 trials/studies) BioAgent Context Research Results LipidsDec 04, 19 · Gelucire pronunciation with meanings, synonyms, antonyms, translations, sentences and more Which is the right way to pronounce the month februari inMay 14, 19 · Gelucire 50/13 as a stabilizer Various commonly used solid lipids and/or liquid lipids (oils) were screened in order to explore the stabilizing potential of Gelucire 50/13 2 Materials and methods 21 Materials Gelucire 50/13 (stearoyl macrogolglycerides), Precirol ATO 5 (glyceryl distearate), Compritol ATO 8 (glyceryl behenate),


Conference On Nanoscience Nanotechnology Frascati December 18


Pharmaceutics Free Full Text The Development And Optimization Of Hot Melt Extruded Amorphous Solid Dispersions Containing Rivaroxaban In Combination With Polymers Html
TGA showed no weight loss, confirming the absence ofGelucire 50/13 (HPMCHPCGel 50/13) were prepared by the same method stated above in the ratio of 111 In this case, the weighed samples of HPMC and HPC were dissolved in 2 ml of absolute ethanol while the Gelucire 50/13 was dissolved in 2 ml of methylene chloride 23 Preparation of the IndHPMCGel 50/13 and IndHPMCHPCGelThe objective of the study was enhancement of dissolution of poorly soluble carvedilol by solid dispersions (SDs) with Gelucire 50/13 using solvent evaporation method The solubility of carvedilol showed linear increase with increasing concentrations of Gelucire indicating A (L) type solubility diagrams



Top Pdf Gelucire 50 13 1library



Thermal And Fractal Analysis Of Diclofenac Gelucire 50 13 Microparticles Obtained By Ultrasound Assisted Atomization Journal Of Pharmaceutical Sciences
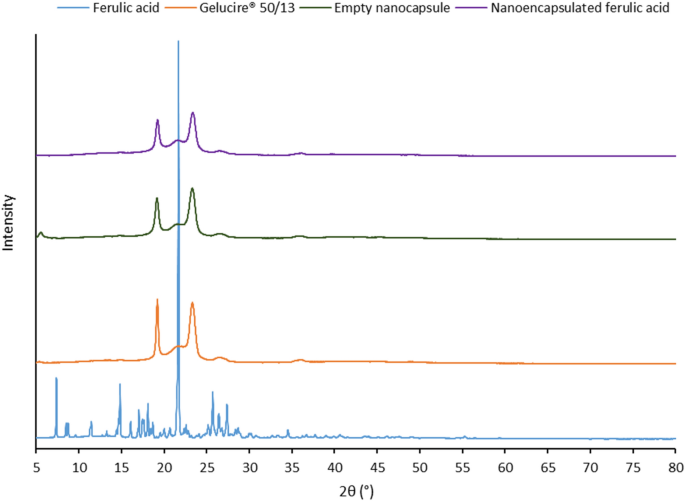
Dec 05, 19 · Cilnidipine is poorly water soluble antihypertensive drug (BCS class II) This study was conducted to increase solubility of drug An attempt was made to formulate fast dissolving tablet of Cilnidipine by solid dispersion using melting method with Gelucire 50/13 The API carrier was taken as 11, 12 and 13 Effect of several variables such as concentration ofThe examined lipid is Gelucire 50/13, which is selected due to higher solubility of ferulic acid in molten Gelucire 50/13 compared to other tested lipids Experimental results show the successful formation of nanoemulsion and subsequently nanoparticle in water with 05 wt % ferulic acidA number of systems were prepared at five compositions (5, 10, , 30 and 40% w/w) of diclofenac/N(2hydroxyethyl) pyrrolidine salt and acidic diclofenac in PEG6000 and Gelucire 50/13, as physical mixtures and as solid dispersions Powder Xray diffractograms for the systems examined show shifted and normal peaks, suggesting that the drug is present inside the samples



Design And Evaluation Of Self Emulsifying Drug Delivery Systems Sedds Of Nimodipine Abstract Europe Pmc



Formulation Studies On Novel Self Solidifying Self Nanoemulsifying Drug Delivery Systems Of Nebivolol Hydrochloride Bentham Science
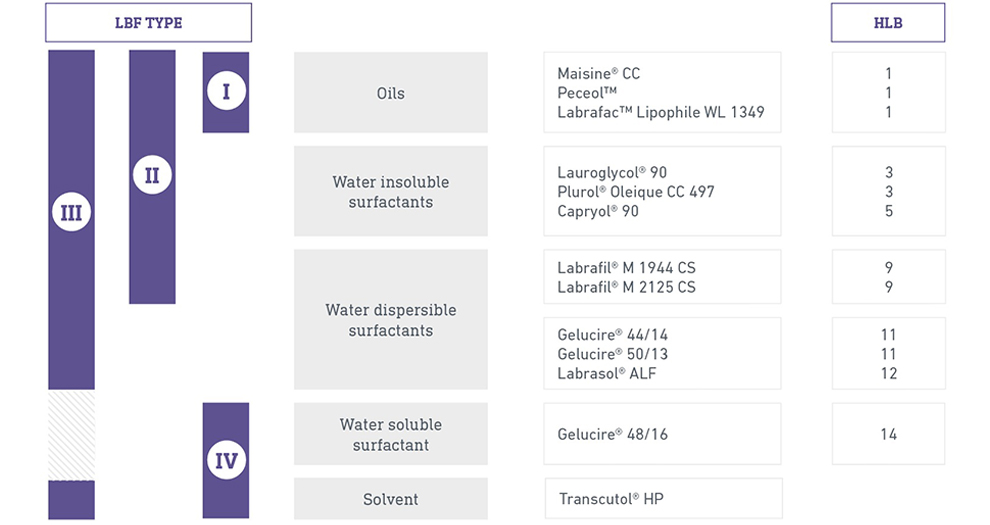
Gelucire 50/13 MinOrder 0 FOB Price USD $ 0000/ 1Our services:ASupply sampleBThe packing also can be according the customers` requirmentCAny inquiries will be replied within 24 hoursDwe provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificateJun 01, 18 · Among the above grades, gelucire 50/13 and 44/14 were selected for review as a lot of research works are published using these two grades 12 Gelucire ® 50/13 It is a nonionic, water dispersible surfactant composed of wellcharacterized PEGesters, aFunctionality Solubilizer for poorlysoluble APIs and bioavailability enhancer Single excipient formulation system selfemulsifies in aqueous fluid



Floating Emulsion Gel Beads On Gelucire For The Sustained Release Of



Improvement In The Dissolution Rate And Tableting Properties Of Cefuroxime Axetil By Melt Granulated Dispersion And Surface Adsorption Sciencedirect
Dec 05, 07 · Gelucire® 44/14 has achieved official USPNF status with pending Food Additive (FCC) status Gelucire® 44/14 is a versatile semisolid lipidic excipient, proven to improve the bioavailability of poorly soluble drugs Gelucire® 50/13 Semisolid bioavailability enhancer and sustained release agent Stearoyl polyoxylglyceridesUSA US08/136,436 USA USA US A US A US A US A US A US A US A US A US A Authority US United States Prior art keywords captopril formulation fatty acid mixture semisolid matrix Prior art date Legal status (The legal status is an assumption and isUtilizing Pluronic F127 and Gelucire 50/13 Solid Dispersions for Enhanced Skin Delivery of Flufenamic Acid



Dissolution Kinetics Of Immediate Release Minitablets Of Cefuroxime Axetil


Lipid Based Formulations Winning Strategy For Oral Bioavailability Enhancement
May 01, 19 · Curcuminresveratrolgelucire 50/13HPβCD (CRGCD) and curcuminresveratrolgelucire 50/13(CRG) SLNs showed a particle size from 100 to 150 nm and were not in the crystalline state per PXRD results MDSC results complimented PXRD results by the absence of melting endotherm of curcumin;Solid dispersions were prepared by dissolving accurately weighed amounts of gelucire 50/13 or gelucire 44/14 or PEG6000 in water and Tinidazole in ethanol After complete dissolution, the aqueous solution of gelucire 50/13, gelucire & PEG6000 were then poured into the ethanolic solution of the TinidazoleMay 24, 11 · Gelucire 50/13 could successfully yield SLN and NLC of various solid lipids, demonstrating its potential to act as a novel stabilizer DSC studies indicated that Gelucire 50/13 interacts with Precirol ATO 5 and this interaction suppresses


Stabilization Of Ferulic Acid In Topical Gel Formulation Via Nanoencapsulation And Ph Optimization Nano Market
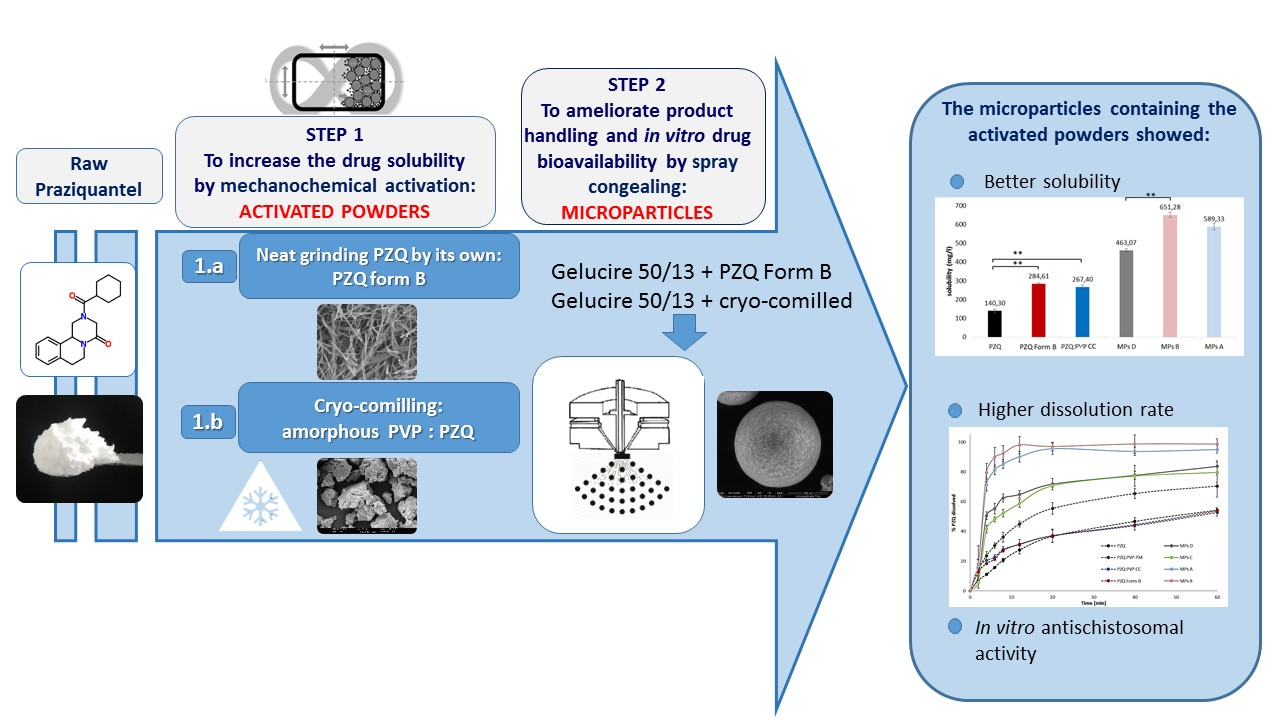


Ijms Free Full Text Combining Mechanochemistry And Spray Congealing For New Praziquantel Pediatric Formulations In Schistosomiasis Treatment Html
Gelucire 50/13 in a ratio of 1 to 175 (drug Gelucire) achieved a drug release of % in 4 hours, a 5fold increase compared to pure carvedilol When incorporating 10% Dαtocopheryl polyethylene glycol succinate (vitamin E TPGS/ TPGS) a higher drug release was observed (%) Parallel artificial membrane permeability assay was used toApplication Water dispersible surfactant, Solubilizer, Bioavailability enhancer, Component of SELF, Matrix for modified release, Multiparticulates;Jun 12, · Gelucirefi 50/13 and Gelucirefi 48/16 were kindly donated by Gattefossè (Milan, Italy) All other chemicals were of analytical grade 22 Preparation of Samples MPs were produced by spraycongealing technology using the Wide Pneumatic Nozzle (WPN) atomizer Initially, the excipient (Gelucirefi 50/13 and Gelucirefi 48/16 in di erent ratio



Pdf American Journal Of Advanced Drug Delivery Immediate Release Formulation Of Valsartan Capsule And Evaluation Of Its Compatibility By Nonthermal Methods American Journal Of Advanced Drug Delivery Ajadd Academia Edu



Simple And Effective Design Of Sedds Formulations Youtube
And Acconon ® C50 or Gelucire 50/13 at ratios , 73, 64, 11, 46 and 37 A batch size of 4 grams was prepared in which each solidifying agent was weighed according to its ratios in the mixtures and then melted in glass scintillation vials on a hot plate until a clear solution (~65ºC) was formed The weighedSimilarly, MDSC data suggests good miscibility of FLT in GMO, GMS, and GEL 50/13 but not in PRE and COM The particle size of drugloaded SLNs prepared from GMO and GMS with GEL 50/13 was found to be 702 ± 54 and 926 ± 85 compared to > 0Jun 16, 16 · The Gelucire 50/13 used as sustained release matrix forming agent in pharmaceutical applications and it was essentially studied by Small and Wide Angle Xray Scattering (SWAXS), Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy according to



Gelucire A Versatile Polymer For Modified Release Drug Delivery System Sciencedirect



Anti Angiogenic Activity Of Uncoated And Em N O Em Carboxymethyl Chitosan Surface Modified Gelucire 50 13 Based Solid Lipid Nanoparticles For Oral Delivery Of Curcumin Researcher An App For Academics
Mar 01, 15 · The present paper reports on physical and thermal properties of polyoxyethylene glycol glycerides (Gelucire 50/13) used as sustained release matrix forming agent in pharmaceutical applications Gelucire 50/13 was essentially studied by Raman and IR spectroscopies according to the temperature and the degree of hydration The hydrationA solid dispersion of Meloxicam (MX), a poorly soluble, non steroidal antiinflammatory drug, and Gelucire 50/13 was prepared by spray drying Spherical microparticles were yielded with smooth surfaces as observed by scanning electron microscopy According to differential scanning calorimetry and powder Xray diffractometry analysis, MX was transformed from the crystallineDrug,gelucire 50/13 and microcrystalline cellulose in aweight ratio of 1510, was markedly rapid and higher than that from the drug powder and the market product (Afinitor®, Novartis Pharmaceuticals) in all dissolution mediums tested from pH 30 to pH 68 Jammula S et al 13studied the effect of Gelucire 50/13 on disso



Central Composite Designed Ezetimibe Solid Dispersion For Dissolution Enhancement Synthesis And In Vitro Evaluation Therapeutic Delivery
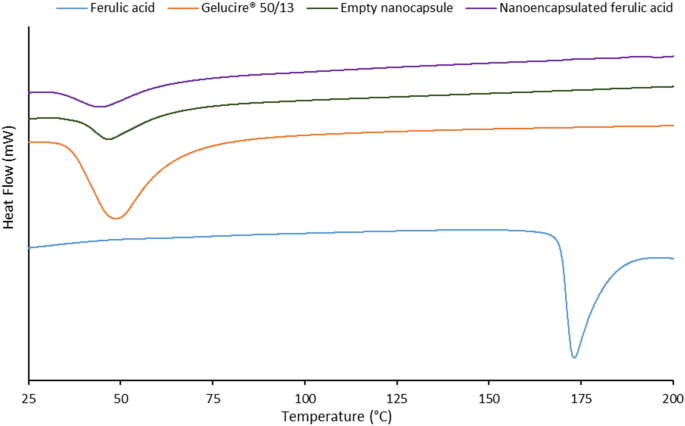


Stabilization Of Ferulic Acid In Topical Gel Formulation Via Nanoencapsulation And Ph Optimization Nano Market
Jul 01, 11 · Purpose To evaluate the ability of Gelucire 50/13 (an amphiphilic lipid excipient) to act as a stabilizer for lipid nanocarriers such as solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) and to establish the ability of Gelucire 50/13 based lipid nanocarriers to improve oral delivery of hydrophobic drugs using repaglinide (RPG) as a model drugWith trypanocidal activity In this work, Gelucire 50/13, a surfactant compound with permeabilityenhancing properties, and silicon dioxide, a drying adjuvant, were employed to produce SDs with UA SDs and physical mixtures (PMs) in different drug/carrier ratios were characterized and compared using differentialGelucire® 50/13 A nonionic waterdispersible surfactant for lipidbased formulations to solubilize and increase oral bioavailability of poorly watersoluble APIs Selfemulsifies in aqueous media forming a fine dispersion, ie, microemulsion (SMEDDS)



Woa1 Composition Google Patents



Vibrational Behavior Of Gelucire 50 13 By Raman And Ir Spectroscopies A Focus On The 1800 1000 Cm 1 Spectral Range According To Temperature And Degree Of Hydration Sciencedirect
May 01, 05 · The study describes the application of a spray‐congealing technique, using a new ultrasound‐assisted atomizer to prepare microparticles of diclofenac/Gelucire 50/13, with the aim to obtain a formulation of enhanced‐release, at 10% w/w drug‐to‐excipient ratio, without any employ of solvent Scanning electron microscopy showed that it was possible to obtain almostGelucire 5013 Subscribe to New Research on Gelucire 5013 inert material derived from hydrogenated foodgrad oils & fats;From 950 to 725 h in F12 and F5, respectively;



Saxs A And Waxs B Diffractogram Of Gelucire 50 13 As A Function Of Download Scientific Diagram
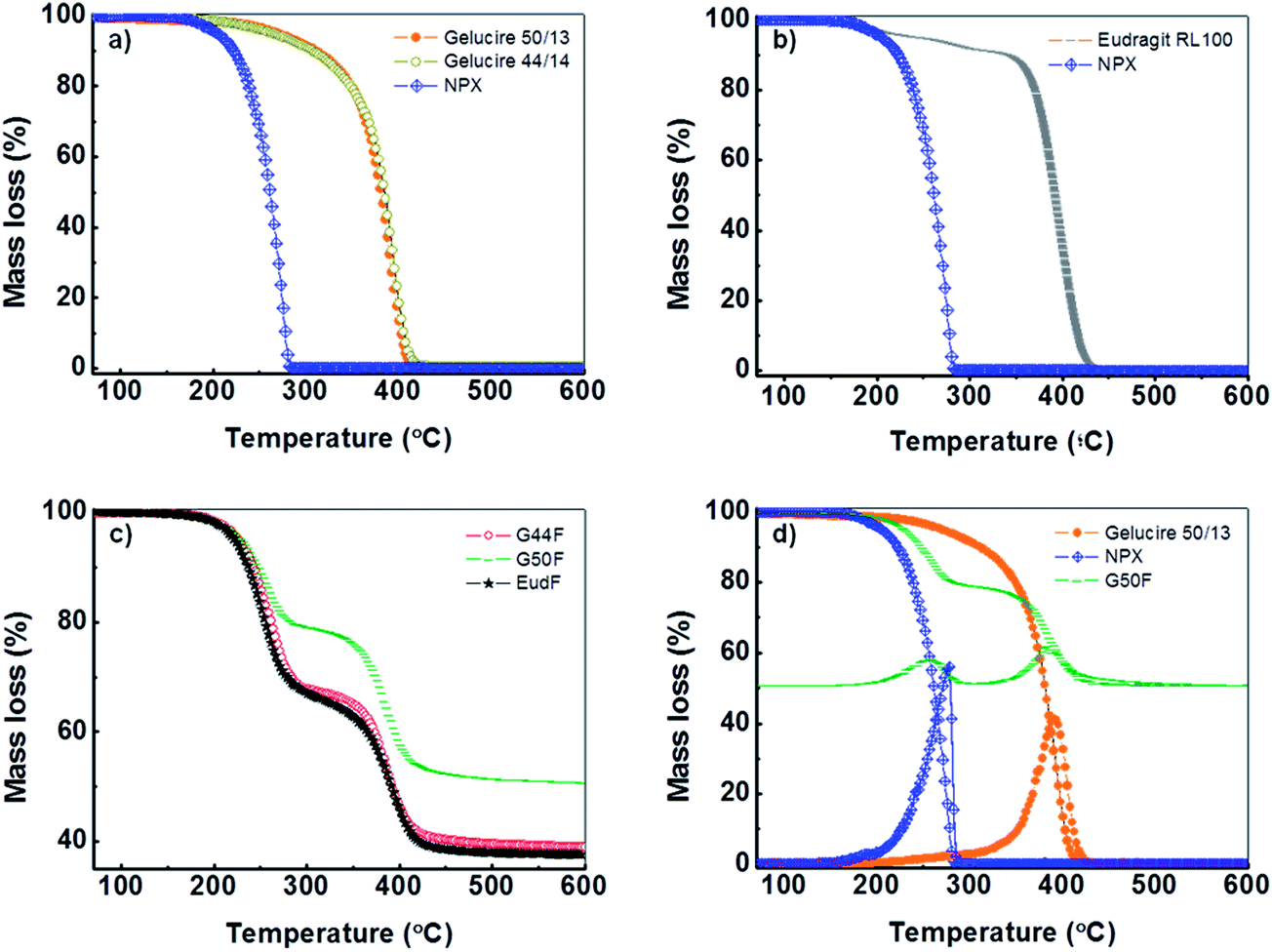


Preparation And Study Of Naproxen In Silica And Lipid Polymer Hybrid Composites Rsc Advances Rsc Publishing
Jun , 12 · Application of Acconon C50 and Gelucire 50/13 as Both Solidifying and Emulsifying Agents for Medium Chain Triglycerides Journal of Excipients and Food Chemicals , Sl, v 3, n 2, p 92, june 12Mar 05, 15 · Gelucire 50/13 is a mixture of mono, di, and triglycerides and mono, diacyl polyethylene glycols For glycerides mixture (approximately % in weight of the sample), the predominant fatty acid chains is the palmitostearic acid (C16 and C18), with approximately the same proportion of mono, di and triglycerides



Gelucire Twitter Search


Gelucire 39 01 As A Matrix For Controlled Release Of Ranitidine Hydrochloride From Floating Granules



Improvement In Solubility Using Poloxamer 1 And Gelucire 50 13 A Case Study Patra Ch Niranjan Swain Suryakanta Panda Tapan Kumar Amazon Com Books



A Factorial Study On The Enhancement Of Ijrpc



13 P Manikandan K P Sampath Kumar By International Journal Of Pharmacy And Pharmaceutical Research Issuu



Dsc Thermograms For Ind Indomethacin Gelu Gelucire 50 13 And Download Scientific Diagram



Improvement In Solubility Using Poloxamer 1 And Gelucire 50 13 A Case Study Patra Ch Niranjan Swain Suryakanta Panda Tapan Kumar Amazon Com Books



Oral Route Bioavailability 2i06 Pharmaceutical Formulation Emulsion



Physicochemical Characterization And Dissolution Properties Of Meloxicam Gelucire 50 13 Binary Systems Topic Of Research Paper In Nano Technology Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science Hub



Sem Images Of Meloxicam A Gelucire 50 13 B Physical Mixture Download Scientific Diagram



Enhancement Of Albendazole Dissolution Properties Using Solid Dispersions With Gelucire 50 13 And Peg Sciencedirect



Saxs A And Waxs B Diffractogram Of Gelucire 50 13 As A Function Of Download Scientific Diagram
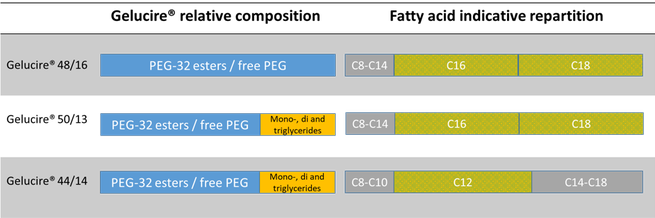


The Gelucire Family Semi Solid Excipients By Gattefosse Pharma Excipients



Figure 8 From A Retinyl Palmitate Loaded Solid Lipid Nanoparticle System Effect Of Surface Modification With Dicetyl Phosphate On Skin Permeation In Vitro And Anti Wrinkle Effect In Vivo Semantic Scholar



Pdf Improvement Of Solubility And Dissolution Rate Of Indomethacin By Solid Dispersions In Gelucire 50 13 And Peg4000 Semantic Scholar



1 Lipid Based Delivery Systems For Oral Administration Lipid Based Delivery Systems Range From Simple Oil Solutions To Complex Mixtures Of Oils Surfactants Ppt Download



Central Composite Designed Ezetimibe Solid Dispersion For Dissolution Enhancement Synthesis And In Vitro Evaluation Therapeutic Delivery



An Investigation Into The Mechanism Of Dissolution Rate Enhancement Of Poorly Water Soluble Drugs From Spray Chilled Gelucire 50 13 Microspheres Journal Of Pharmaceutical Sciences



Figure 5 From Enhancement Of Solubility And Dissolution Rate Of Loratadine With Gelucire 50 13 Semantic Scholar



Reported Literature On Gelucire 50 13 Download Scientific Diagram
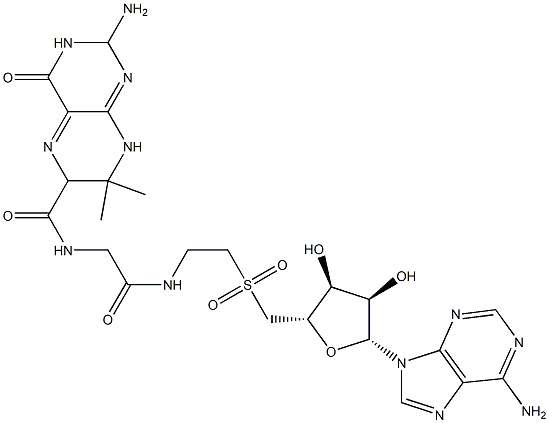


Gelucire 50 13 Cas 05 8



Document


View Of Enhancement Of Dissolution Rate Of Hydrochlorothiazide International Journal Of Pharmacy And Pharmaceutical Sciences



Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents



Pdf Improvement In The Dissolution Rate And Tableting Properties Of Cefuroxime Axetil By Melt Granulated Dispersion And Surface Adsorption Sarwar Beg Academia Edu



Pdf Preparation Of Solid Dispersion Of Everolimus In Gelucire 50 13 Using Melt Granulation Technique For Enhanced Drug Release Semantic Scholar



Poloxamer 407 Basf Bioz Ratings For Life Science Research
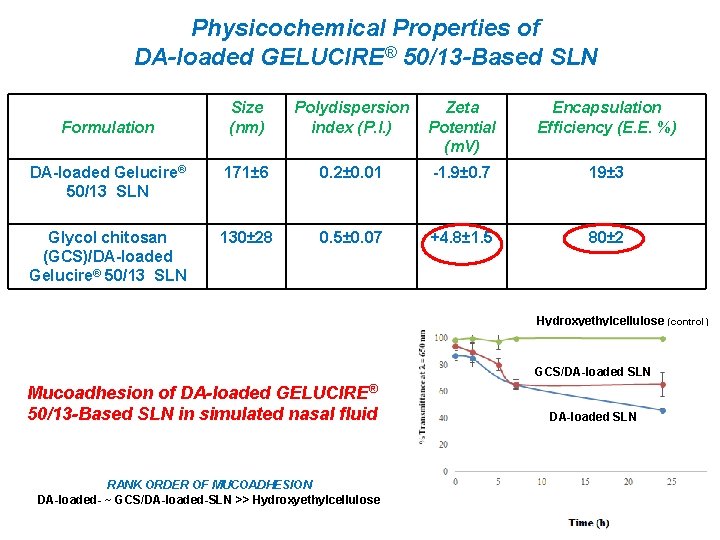


Conference On Nanoscience Nanotechnology Frascati December 18



Characterization Of Carbamazepine Gelucire 50 13 Microparticles Prepared By A Spray Congealing Process Using Ultrasounds Passerini 02 Journal Of Pharmaceutical Sciences Wiley Online Library
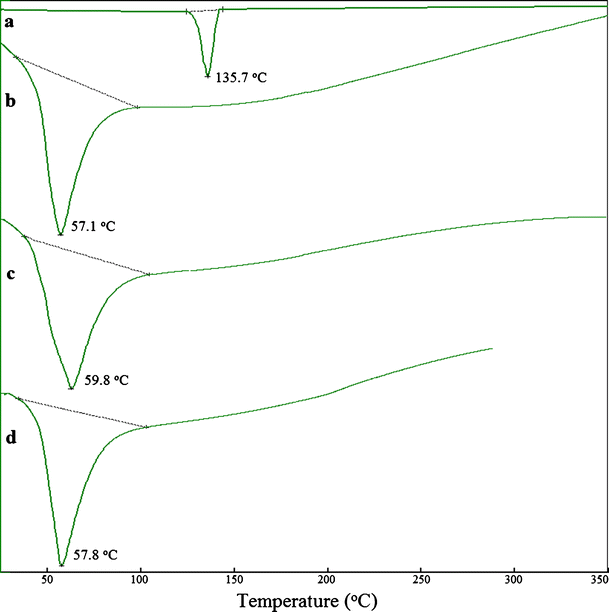


Enhancement Of Solubility And Dissolution Rate Of Loratadine With Gelucire 50 13 Springerlink
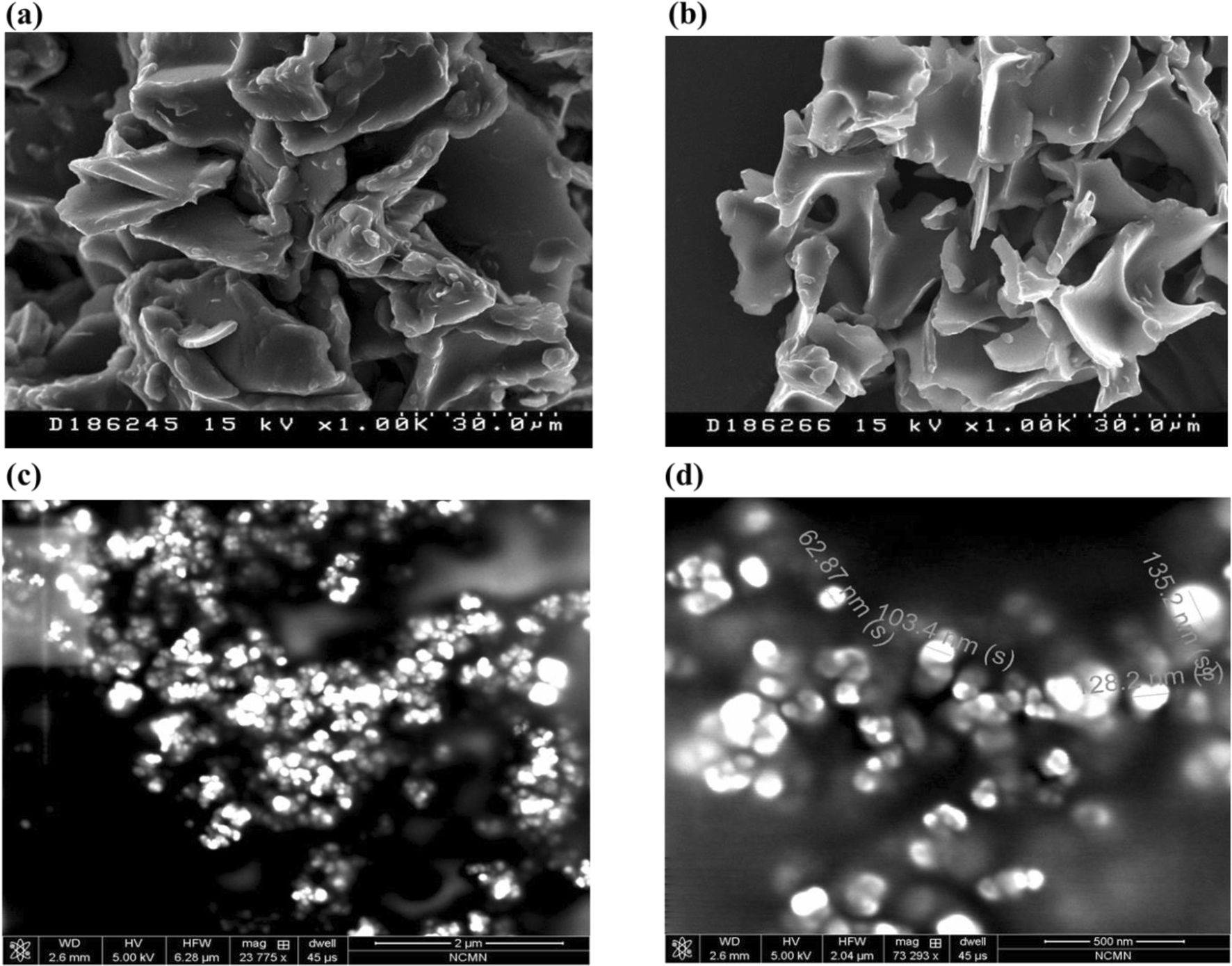


Figure 6 Preparation Characterization And In Vitro Evaluation Of Curcumin And Resveratrol Loaded Solid Lipid Nanoparticles Springerlink


Journal Club Seminar On Authorstream
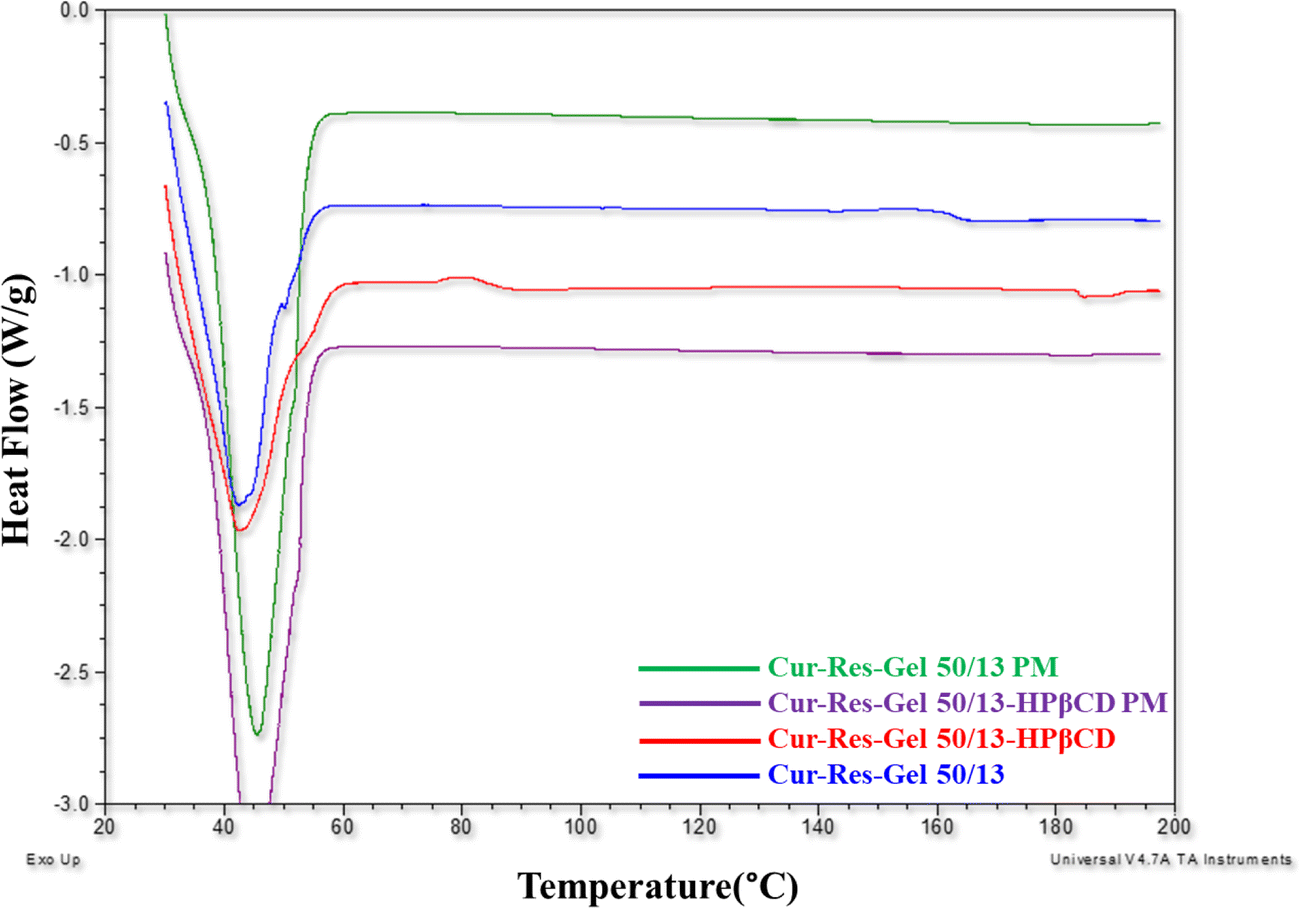


Figure 8 Preparation Characterization And In Vitro Evaluation Of Curcumin And Resveratrol Loaded Solid Lipid Nanoparticles Springerlink
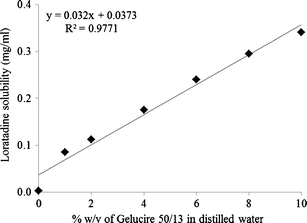


Enhancement Of Solubility And Dissolution Rate Of Loratadine With Gelucire 50 13 Springerlink



Gelucire 50 13 Pharma Excipients



Dsc Thermograms Of Sa Gelucire 50 13 And 39 01 Download Scientific Diagram
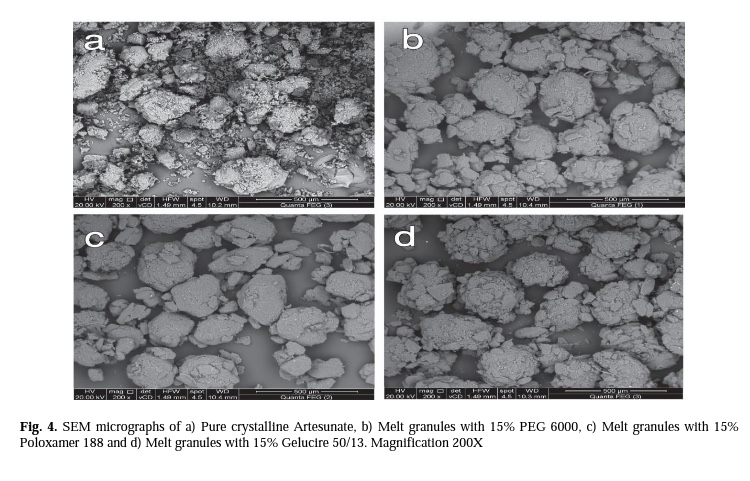


Enhancing Dissolution Of Artesunate From Immediate Release Tablets Using A Green Granulation Technique Pharma Excipients



Journal Of Applied Pharmaceutical Science


Conference On Nanoscience Nanotechnology Frascati December 18



05 8 Gelucire 50 13 C23h32n12o8s Formula Nmr Boiling Point Density Flash Point



0 件のコメント:
コメントを投稿